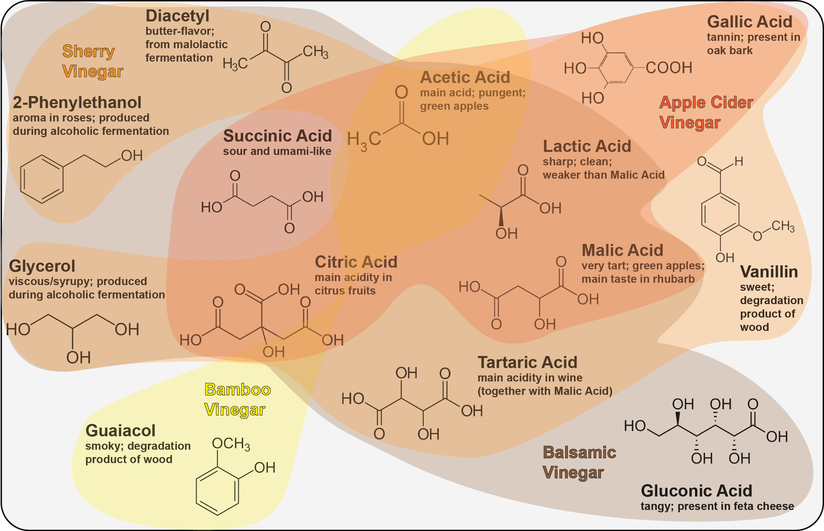
Vinegar, the fate of alcohol according to Harold McGee1, is one of the main sources of acidity in the culinary world. Joining the ranks of other fermented food items such as chocolate, cheese or soy sauce, vinegar is produced by a process called acetic acid fermentation or acetification. And while there is still innovation in the whole process of acetification2, the history of vinegar goes way back to the ancient Romans and even the Babylonians 4000 BCE1. To find out if we can do something truly novel with vinegar in the cocktail world we first have to dive deep into the microbial world of acetification.
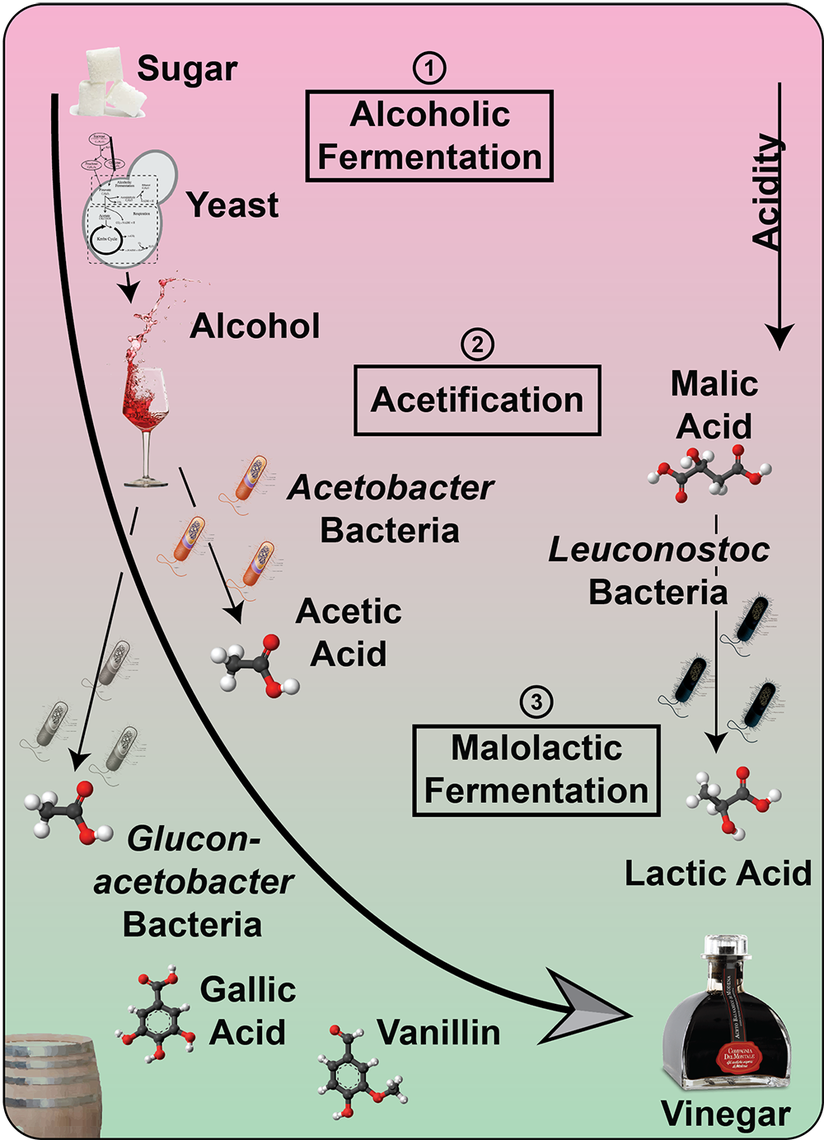
And then there is acetic acid itself, the key player in acetification. Acetic acid does not only have a taste but also a very distinct smell1. Yet it comes in two forms, undissociated and dissociated. The more acidic the vinegar is (especially from different acids) the more undissociated acetic acid is present1. As you can smell only the undissociated acetic acid, a stronger aroma can be detected from vinegars which are more acidic. Thus, wine vinegars which are rich in tartaric acid will have a stronger acetic acid aroma than, for instance, malt vinegar6. Thanks to its chemical nature, acetic acid (and thereby vinegar) can extract aromatic compounds substantially better from herbs and spices than water-based solutions can, making it a prime candidate for infusions1.
Vinegar, in the form of shrubs, has recently resurfaced in the world of drinks7. And rightly so, as it offers a number of benefits over its acidic competitor, citrus juice. In contrast to citrus juice, vinegar is supremely shelf-stable7 (thank you acetic acid!) and does not have to be juiced for every cocktail. Additionally, vinegars have a lot more potential for flavor complexity and drinks shaken with vinegar instead of citrus juice remain clear8. Acetic acid is also a lot more powerful than citrus juice, which is why Kelley Slagle coined the general rule to add three times less vinegar to drinks than you would for citrus juice9. However, until now the main acids used in drinks are citric acid (from citrus fruits), malic acid (limes, apples and grapes) and tartaric acid (vermouth and champagne)9. Bartenders all over the world are even experimenting with chemically pure acids, either those mentioned above or others such as succinic acid or lactic acid10,11. By adding the pure acid to a drink, you are able to control exactly one factor without bringing in all the (potentially unwanted) color, flavor and texture which you would get from, say, a lemon.
The extreme diversity of different vinegars yields ample room for innovation. Naomi Schimek of the Spare Room uses coconut vinegar (yielding a slightly yeasty flavor12) for her tiki-inspired shrub13; at the Pitt Cue Co in London pickle juice (which contains a lot of vinegar) is used for their cocktail Big Mac ’n’ Rye14 and Pok Pok’s Alex Mirkin uses honey vinegar for their Pok Pok Ny’s Hunny15. In principle anything that contains alcohol can be converted into vinegar. This even opens up the possibility to convert spirits such as rum or whiskey into vinegar. Here, the only issue is the high alcohol content which would immediately kill the acetic acid bacteria. Thus, one would either need to dilute the spirit to about 20 proof or cook off the alcohol (for about two hours16), both with immediate downsides to flavor. With the panoply of vinegars just emerging at the forefront of cocktails, surely many interesting innovations such as rum vinegar or vinegar based on wines made from foraged wild local plants are still to come and enrich life at the bar.
1) https://books.google.ch/books/about/On_Food_and_Cooking.html?id=bKVCtH4AjwgC&redir_esc=y
2) https://www.hindawi.com/journals/tswj/2014/394671/
3) https://link.springer.com/article/10.1007%2Fs00253-015-6659-1
4) http://www.sciencedirect.com/science/article/pii/S0308814608009898?via%3Dihub
5) http://www.sciencedirect.com/science/article/pii/S0308814608000800
6) http://pubs.acs.org/doi/full/10.1021/jf021180u
7) http://www.nytimes.com/2011/10/12/dining/vinegar-cocktails-are-making-the-rounds.html
9) https://www.diffordsguide.com/encyclopedia/62/cocktails/vinegar-and-vinegar-in-cocktails
10) http://www.cooksscience.com/articles/story/balance-of-sour/
11) https://books.google.ch/books/about/Liquid_Intelligence.html?id=UrPmngEACAAJ&redir_esc=y
12) https://recipes.howstuffworks.com/how-vinegar-works1.htm
rn13) http://articles.latimes.com/2012/may/26/food/la-fo-shrub-cocktails-20120526
15) http://www.seriouseats.com/recipes/2012/08/pok-pok-hunny-cocktail-grapefruit-drinking-vinegar-tequila.html
16) https://en.wikipedia.org/wiki/Cooking_with_alcohol#Alcohol_in_finished_food