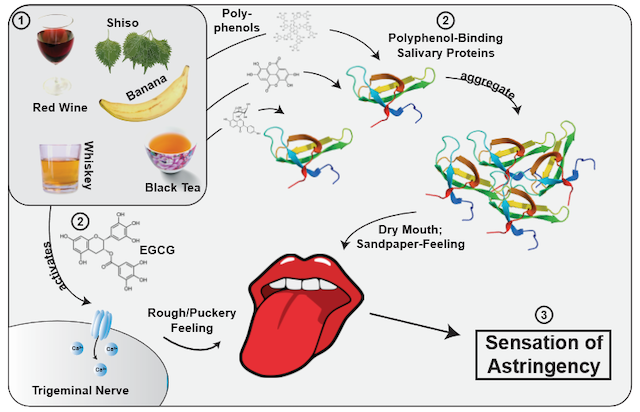
A slightly unripe banana. A glass of Cabernet Sauvignon. The feeling of your mouth drying up as you take a sip from your cup of black tea. Astringency. An effect produced by polyphenols, metals, and acids, as well as dehydrating agents1, the familiar puckering feeling is still at the forefront of scientific research. However, the question of its working mechanism—or rather, mechanisms—is still unsettled.
Contrary to intuition, astringency is actually not a taste, but rather a tactile perception2. It’s similar to the effect experienced when tasting Sichuan peppercorns3. To understand this phenomenon, scientists blocked the taste nerves of human subjects and found that their “taste” of astringency was undiminished2. Depending on the specific polyphenol (a category which encompasses tannins from wines and oak as well as catechins present in tea), the perception is different. Some polyphenols bind to proteins in the saliva, leading to the loss of saliva’s the lubricating effect1. This is the cause behind the drying effect of astringent polyphenols, and is complimented by a sandpaper-like feeling created by the friction of the precipitated protein-polyphenol complexes4. However, there are polyphenols which do not bind to salivary proteins at all. For example, EGCG (the main polyphenol in tea) directly activates the trigeminal nerve, which is normally responsible for facial muscles2. All this to say, we “feel” the tannins in a red wine more than we actually “taste” them.
As anyone that has ever tasted a very astringent wine can testify, the perception of astringency needs some time to fully develop (about 15 seconds) and lingers for far longer (about 5 minutes) 5. To differentiate between two astringent beverages, a wine taster might be tempted to wash away the astringent perception with oil or milk, or by eating crackers. While this does work very effectively, the astringency of the next drink will now be under-experienced. The best solution, therefore, is to wait, and maybe drink some water6. Since astringency is very much about saliva, people with a higher saliva flow rate and a higher salivary protein concentration have a much weaker perception of astringency7,8.
Astringency is influenced by many factors. Increasing the alcohol concentration of a cocktail decreases the intensity of astringency because of an increase in viscosity9. Likewise, an increase in temperature will lower the viscosity and thereby increase the intensity of the astringency9. Even your astringent source material can be highly variable, as we perceive different polyphenols with different astringency intensities even if they are at the same concentration10. On top of that, the polyphenols themselves are rarely at the same concentration. Oolong tea for example, has a lower polyphenol content when it’s cultivated at higher altitudes10.
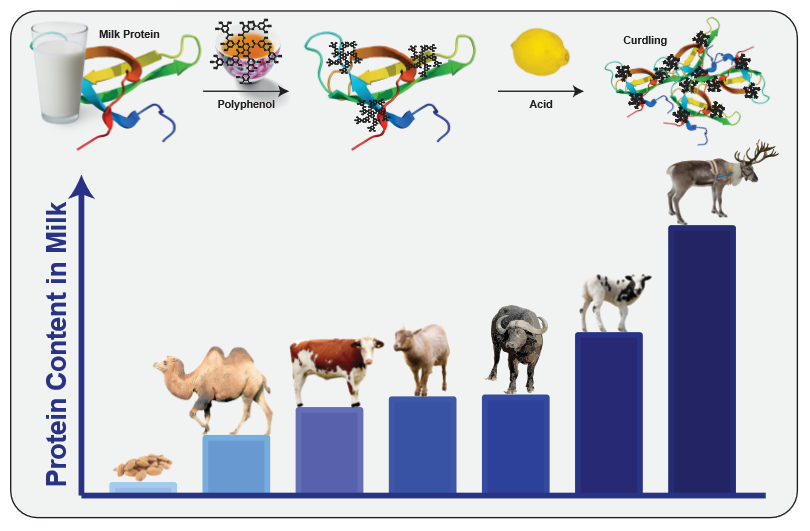
Spearheaded by Dave Arnold from Booker and Dax11, milk- or egg-washing astringent infusions—such as black tea vodka—have become reliably successful ways to modify astringency. In the case of milk-washing, the milk proteins (most notably casein) bind to the astringent polyphenols from tea12. Once acid is added to the mixture, they begin to curdle, and can be filtered out together with the bound polyphenols. As noted above, polyphenols can also come from oak, such as in barrel-aged spirits, and can be removed by the same technique. This not only removes astringency from the cocktail, but also leaves something behind.
Whey proteins (mostly beta-lactoglobulin) also bind polyphenols, but they don’t curdle in the presence of acid. Therefore, they can act as foam stabilizers in milk-washed cocktails13. Apart from that, the milk used for washing contains far more than just protein: it also contains lactose and fats, which can impart some flavor to the final product14. Although cow milk got the whole “milk-washing” thing started, there is no inherent reason to limit the technique to that. In fact, Gabriella Mlynarczyk from the Birch restaurant in Los Angeles uses goat milk to wash spirits, which she found gave her the best results in terms of flavor and clarification speed14. On a more exotic note, Paul Bradley of Gold on 27 even tried to use camel milk in one of his cocktails14.
Apart from flavor, one of the most notable differences between different kinds of milk is their protein content. While camel milk has slightly less protein than cow milk, the milk of goats, buffalos, sheep and reindeers contain progressively more protein15,16. Thus, by mixing them with spirits, you could achieve a more thorough wash with interesting flavors; alternately, you could use less milk, and thereby keep your milk-washed spirit at a higher alcohol concentration. Other factors also come into play: as goat milk is more alkaline than cow milk17, it needs more added acidity to bring it to curdling (which might lend a citrusy flavor if you use lemons to achieve that). However, not every milk is suited for this. Different nut milks, for instance, typically do not contain casein18, and therefore cannot bind polyphenols. In addition, scientific studies found that the slightly sweet dietary fiber polydextrose efficiently bound to polyphenols and masked the astringency of beverages19, making it an interesting candidate for a cocktail ingredient.
Far from being a villain, astringency can be a wonderful taste to be savored. Even though polyphenols can be healthy for us, the ability of our salivary proteins to bind polyphenols is actually a clever protection mechanism, as some polyphenols can inhibit digestive enzymes4. Bartenders even have started to experiment with new ways to add astringency to their cocktails with ingredients such as the Japanese herb Shiso20, the Middle Eastern spice sumac21, or through creating an infusion with tea to generate new and interesting flavors. Only if the astringency proves to be overpowering, or is an unwanted feature in the final drink, should one feel the necessity to remove it by washing with milk or eggs.
That being said, I do wonder what a cocktail made with reindeer milk would taste like.
Sources:
1) https://www.ncbi.nlm.nih.gov/pubmed/22515235
2) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4064959/
3) https://www.smithsonianmag.com/science-nature/why-szechuan-peppers-make-your-lips-go-numb-5668606/
4) https://onlinelibrary.wiley.com/doi/10.1111/jtxs.12022/pdf
5) https://www.ajevonline.org/content/37/3/184
6) https://www.sciencedirect.com/science/article/pii/S0950329309001207
7) https://www.sciencedirect.com/science/article/pii/0950329394900086
8) https://europepmc.org/abstract/AGR/IND23286005
9) https://www.sciencedirect.com/science/article/pii/S0924224414001654
10) https://www.sciencedirect.com/science/article/pii/S1021949816301417
11) https://books.google.ch/books/about/Liquid_Intelligence.html?id=UrPmngEACAAJ&redir_esc=y
12) https://www.tandfonline.com/doi/full/10.1080/87559129.2017.1377225
13) https://link.springer.com/content/pdf/10.1007/978-3-642-59116-7_6.pdf
14) https://www.cooksscience.com/articles/story/the-key-to-crystal-clear-cocktails-milk-really/
15) https://www.milkfacts.info/Nutrition%20Facts/Nutrient%20Content.htm
16) https://www.foodnutritiontable.com/nutritions/nutrient/?id=1194
17) https://pdfs.semanticscholar.org/8f2a/3ec1d4135ad9fb747b4d9fbd55dbb400a895.pdf
18) https://time.com/3677300/almond-milk-nutrition/
19)https://www.researchgate.net/publication/229102662_Alternatives_to_reduce_the_bitterness_astringency_and_characteristic_flavour_of_antioxidant_extracts
20) https://www.thekitchn.com/straight-up-shiso-mojitos-51128
21) https://food52.com/blog/14546-why-you-should-be-adding-sumac-to-your-cocktails