The common denominator of most modern cocktails is their use of citrus juice. Acidifying and brightening up drinks, citrus juice has the characteristics of a staple ingredient which we tend not to think too much about. For instance, one of the iron laws of citrus juice which most people tend to follow without thinking is: Use your citrus juice as fast as possible after juicing as it deteriorates quickly. Commonsensical, pithy, practical advice, but also not quite right.
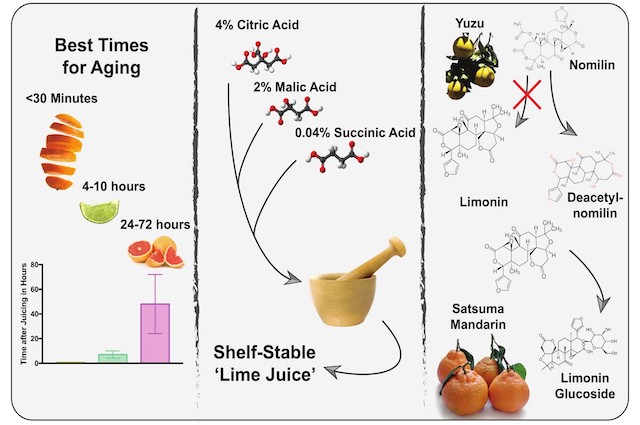
Some years ago, Dave Arnold and Kevin Liu found that people in blind tastings actually preferred ‘aged’ lime juice over fresh lime juice1,2. While aging may not carry the same weight here as in the aging of whiskey, four to 10 hours of aging gives lime juice a slight upper hand over its fresh version. After 10 hours, the familiar pattern of deterioration emerges and you should prepare new lime juice. Tasting notes described four-hour-old lime juice as ‘more mellow’ and ‘well-balanced’ compared to its fresh counterpart and after the blissful first 10 hours, bitterness increased perceptibly with an accompanying loss of flavor2,3. The question remains though why and how a process called enzymatic bittering is helping lime juice to shine in its early hours?
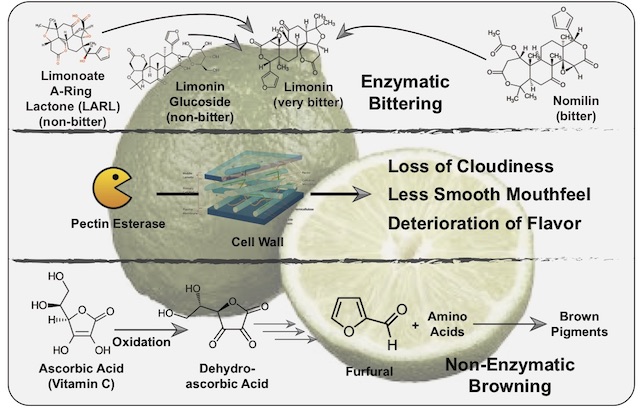
Terpenoids encompass many molecules present in citrus, such as the key citrus flavors limonene (mostly present in the peel) and citronellal (also the major aroma compound in kaffir lime leaves)2,4. One group of terpenoids we are interested in now are the limonoids, a class of 36 phytochemicals with six bitter members (limonin, nomilin, ichangin, obacunoate, nomilinate and deoxylimonate)5. Of these, we will mainly focus on limonin and nomilin. And while both of these compounds are bitter-tasting, limonin has a drastically lower threshold with some tasters being able to pick it up at 0.5 ppm (parts per million)3. Whereas limonin is the bitter taste determinant of blood orange, bitter precursors such as nomilin and limonoate A-ring lactone (LARL) are present in citrus fruits such as limes5. Following mechanical damage, for instance by juicing, enzymes come into contact with nomilin and LARL, over time converting them into the very bitter limonin. More intense contact, such as through excessive rolling of limes to induce inner damage, intensifies this bitter taste3. This is especially pronounced as nomilin and limonin increase each other’s bitterness through synergy5. On top of this enzymatic process, called enzymatic bittering, other enzymes such as limonoid glucoside beta-glycosidases can be liberated from damaged citrus seeds and convert the tasteless glucosides of nomilin/limonin into the bitter-tasting free versions.
 A visual demonstration of the aging effects on enzymatic bitters in citrus.
A visual demonstration of the aging effects on enzymatic bitters in citrus.
The reason why enzymatic bittering is improving the flavor of lime juice (at least for several hours after juicing, until the bitter taste becomes too pronounced), is found in the phenomenon of taste suppression. Increased levels of bitterness are able to partially suppress basic tastes such as acidity2. And as anyone who has ever tasted citrus juice on its own can attest to, excessive acidity can prevent you from fully enjoying the taste panoply of citrus juice. Therefore, a mellowing of the acidic taste by an increase in bitterness (thanks to enzymatic bittering) can allow for a deeper appreciation of the flavor of the respective citrus juice. Of course an excess of bitterness (after the first 10 hours subsequent to juicing) then contributes to the quality deterioration of citrus juice.
Apart from enzymatic bittering, other chemical processes are happening in citrus juice. Enzymes such as pectin esterase degrade the pectins which make up part of the cell wall of plant cells7. This leads to less cloudiness, a different mouthfeel and, ultimately, a deterioration of flavor. And while freezing fresh citrus juice may slow down these enzymes, they are not inactivated2. Additionally, the oxidation of ascorbic acid (vitamin C) over time kickstarts a process known as non-enzymatic browning8. Through this process, degradation products of ascorbic acid (furfurals and other carbonyl compounds) react with each other and/or amino acids, creating off-flavors during this process and contributing to the decline in citrus juice quality over longer time periods. The phenomenon of citrus juice aging does not hold true for orange juice, which quickly turns perceptibly bitter and should be consumed within 30 minutes after juicing2. Grapefruit juice however, which is bitter to start with, is not so fragile and profits from an aging period of one to three days (intensifying its grapefruit flavor).
Using pure acids or vinegars instead of citrus juice circumvents the variability and eventual quality decline described here. To emulate lime juice (which in terms of flavor is arguably more complex than lemon juice), Dave Arnold combines citric acid, malic acid and a tiny bit of succinic acid to create an acid solution which, compared to lime juice, is supremely shelf-stable9,10. However, there are other solutions. Citrus ichangensis X C. reticulata var austera (commonly known as the Asian citrus fruit yuzu) is producing a special enzyme called nomilin deacetylase which converts nomilin to the non-bitter compound deacetylnomilin and thereby prevents the build-up of bitter limonin from nomilin upon juicing5. Even though this has not been specifically investigated, it would be interesting to see if yuzu juice therefore has a longer shelf-life until oxidation processes kick in. Additionally, crossing this plant with other citrus fruits (or using more advanced techniques) might spread this potentially favorable trait to other citrus fruits. A similar process occurs in the Japanese satsuma mandarin (Citrus unshiu), which keeps limonin in its non-bitter glucoside form with the help of the enzyme limonoid UDP-glucosyltransferase and thereby substantially delays the development of bitterness in its juice11. By utilizing both the subtle production of bitterness during the short-term aging process for flavorful juices as well as citrus species which have a delayed bitterness development for the production of shelf-stable citrus juices, we can have our cake and eat (or rather drink) it too.
1) https://www.cookingissues.com/2010/10/01/fresh-lime-juice-wtf/
3) https://blog.doingsciencetostuff.com/2013/11/23/lemon-vs-aged-lemon/
4) https://www.researchgate.net/publication/236200539_Determination_of_Limonin_and_Nomilin_contents_in_different_Citrus_cultivars_using_high_performance_liquid_chromatography
5) https://link.springer.com/content/pdf/10.1007/978-1-4615-4693-1_9.pdf
7) https://edis.ifas.ufl.edu/fe834
8) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4190239/
9) https://www.cooksscience.com/articles/story/balance-of-sour/
10) https://books.google.ch/books/about/Liquid_Intelligence.html?id=UrPmngEACAAJ&redir_esc=y
11) https://onlinelibrary.wiley.com/doi/10.1016/S0014-5793(00)01275-8/full




